Minimise Side Effects with Spaceoar Rectal Spacer
Radiation Therapy is an effective curative treatment for Prostate Cancer. Technological advances like Intensity Modulated Radiotherapy (IMRT), Stereotactic Body Radiation Therapy (SBRT) and Proton Beam Therapy (PBT) have allowed high doses of radiation to be more focused on the prostate while avoiding the nearby healthy tissues.
The rectum is located right next to the prostate, so it is inevitable that a very small part of it would still receive high doses of radiation. There is a small risk of bleeding from the back passage, months to years after radiotherapy, because of this. That is where Spaceoar Rectal Spacer comes in, literally and figuratively, to make Prostate Radiotherapy even safer.
What is Spaceoar Rectal Spacer and how does it work?
Spaceoar Rectal Spacer is made from a soft gel-like synthetic material that consists of 90% water and is safe to use in the body.
It is placed in between the rectum and the prostate, increasing the distance between the 2 organs by approximately 1 cm. The space created, essentially pushes the rectum away from the radiotherapy high dose region, vastly reducing high radiation dose to the rectum (refer to Figure 1).
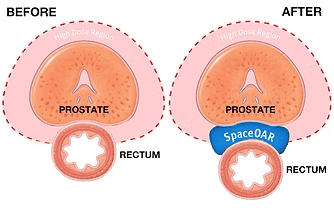
Figure 1: Hydrogel Placement (Boston Scientific, 2022)
The gel will have a fixed shape and size for 3 months, before liquifying and being naturally absorbed and excreted by the body within 6 months.
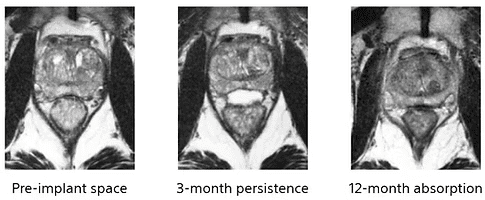
Figure 2: MRI of a patient with SpaceOAR Hydrogel (Mariados N et al., 2015)
Less exposure to high dose radiation means less risk of long-term side effects from radiation. This has been demonstrated in a clinical trial whereby patients receiving prostate radiotherapy with the Spaceoar Rectal Spacer had significantly a 75% reduction in mild and above rectal bleeding compared to those who did not. Notably, there were no cases of moderate and above rectal bleeding in patients who had Spaceoar Rectal Spacer (refer to Figure 3)

Figure 3: Results from a clinical trial on the usage of SpaceOAR: Reduction in
rectal bleeding (Mariados N et al., 2015)

Figure 4: Hydrogel Placement (Boston Scientific, 2022)
The Spaceoar Rectal Spacer Procedure
It can be done as a minimally invasive office procedure / day case under local anaesthesia, and typically takes less than an hour. Guided by an ultrasound probe, sterile saline is injected to open up the space between the rectum and the prostate, followed by injection of the Spaceoar Rectal Spacer gel into the space.
What happens after the Spaceoar Rectal Spacer placement?
Hospital stay is not required and the patient can go home soon after the procedure. A sensation of fullness in the back passage may be felt for a day or two, but this is temporary. Return to normal activities can be expected soon after the procedure and there is virtually no downtime. The CT and and MRI scans for radiotherapy planning can then be done a week after the procedure.
In summary,
Spaceoar Rectal Spacer is clinically shown to minimise urinary, sexual and bowel side effects and protect quality of life for prostate cancer patients undergoing radiation therapy. In addition, it is also
It is minimally-invasive
A brief outpatient prodedure
Performable under local anaesthesia
Shown by Clinical Trial to reduce long term rectum side effects
Included in Internationally Recognized Prostate Cancer Guidelines
Eligible for Full Insurance Coverage
Boston Scientific. (2022, November 21). SpaceOAR Vue™ Hydrogel: Radiopaque Perirectal Spacer for Radiation Therapy. https://www.bostonscientific.com/en-US/products/hydrogel-spacers/spaceoar-vue-hydrogel/healthcare-professionals-resources.html
Mariados, N et al. Hydrogel spacer prospective multicenter randomized controlled pivotal trial: dosimetric and clinical effects of perirectal spacer application in men undergoing prostate image guided intensity modulated radiation therapy. Int J Radiat Oncol Biol Phys. 2015 Aug 1;92(5): 971–7.
Hamstra DA, Mariados N, Sylvester J, et al. Continued benefit to rectal separation for prostate radiation therapy: Final results of a phase III trial. Int J Radiat Oncol Biol Phys. 2017 Apr 1;97(5):976-85.
National Comprehensive Cancer Network (2022, September 16). Clinical Practice Guindelines in Oncology: Prostate Cancer Version 1.2023
